what is the ability of a substance to dissolve in another substance?

Example for a dissolved solid (left)

Germination of crystals in a four.2 M ammonium sulfate solution. The solution was initially prepared at 20 °C and so stored for 2 days at four °C.
In chemical science, solubility is ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite holding, the disability of the solute to grade such a solution.
The extent of the solubility of a substance in a specific solvent is more often than not measured as the concentration of the solute in a saturated solution, i in which no more solute can be dissolved.[ane] At this betoken, the two substances are said to be at the solubility equilibrium. For some solutes and solvents there may exist no such limit, in which case the 2 substances are said to be "miscible in all proportions" (or just "miscible").[2]
The solute can exist a solid, a liquid, or a gas, while the solvent is ordinarily solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,[3] and a solid or liquid can be "dissolved" in a gas only by passing into the gaseous state first.
The solubility mainly depends on the composition of solute and solvent (including their pH and the presence of other dissolved substances) too as on temperature and pressure. The dependency can often be explained in terms of interactions between the particles (atoms, molecules, or ions) of the two substances, and of thermodynamic concepts such every bit enthalpy and entropy.
Under sure atmospheric condition, the concentration of the solute tin exceed its usual solubility limit. The result is a supersaturated solution, which is metastable and will rapidly exclude the backlog solute if a suitable nucleation site appears.[4]
The concept of solubility does not utilise when there is an irreversible chemical reaction between the two substances, such equally the reaction of calcium hydroxide with muriatic acid; even though one might say, informally, that one "dissolved" the other. The solubility is also not the same every bit the rate of solution, which is how fast a solid solute dissolves in a liquid solvent. This property depends on many other variables, such every bit the physical form of the two substances and the way and intensity of mixing.
The concept and measure of solubility are extremely important in many sciences besides chemistry, such as geology, biology, physics, and oceanography, as well as in engineering, medicine, agriculture, and even in not-technical activities like painting, cleaning, cooking, and brewing. Most chemical reactions of scientific, industrial, or practical interest only happen later the reagents have been dissolved in a suitable solvent. Water is by far the most common such solvent.
The term "soluble" is sometimes used for materials that can form colloidal suspensions of very fine solid particles in a liquid.[5] The quantitative solubility of such substances is generally non well-defined, however.
Quantification of solubility
The solubility of a specific solute in a specific solvent is generally expressed every bit the concentration of a saturated solution of the two.[1] Whatever of the several means of expressing concentration of solutions can be used, such as the mass, volume, or amount in moles of the solute for a specific mass, book, or mole corporeality of the solvent or of the solution.
Per quantity of solvent
In particular, chemic handbooks will oftentimes express the solubility of a substance in a liquid as grams of solute per decilitre (100 mL) of solvent (thousand/dL); or, less usually, as grams per litre (g/50). The quantity of solvent can instead exist expressed in mass, every bit in one thousand/100g" or g/kg. The number may be expressed as a percentage in this case, and the abbreviation "w/w" may be used to indicate "weight per weight".[6] (The values in g/L and g/kg are practically the same for water, but not for other solvents.)
Alternatively, the quantity of solute can be expressed in moles instead of mass; if the quantity of solvent is given in kilograms, the value is the molality of the solution (mol/kg).
Per quantity of solution
The solubility of a substance in a liquid may as well exist expressed as the quantity of solute per quantity of solution, rather than of solvent. For example, following the common practice in titration, it may be expressed as moles of solute per litre of solution (mol/50), the molarity of the latter.
In more specialized contexts the solubility may exist given by the mole fraction (moles of solute per total moles of solute plus solvent) or by the mass fraction at equilibrium (mass of solute per mass of solute plus solvent), both adimensional numbers between 0 and 1 which may be expressed as percentages.
Liquid and gaseous solutes
For solutions of liquids or gases in liquids, the quantities of both substances may be given volume rather than mass or mole amount; such as litre of solute per litre of solvent, or litre of solute per litre of solution. The value may be given as a percentage, and the abbreviation "v/v" for "book per volume" may be used to indicate this option.
Conversion of solubility values
Conversion between these various means of measuring solubility may non be trivial, since it may require knowing the density of the solution — which is often not measured, and cannot exist predicted. While the total mass is conserved by dissolution, the last volume may be different from both the volume of the solvent and the sum of the ii volumes.[vii]
Moreover, many solids (such as acids and salts) will dissociate in non-trivial ways when dissolved; conversely, the solvent may form coordination complexes with the molecules or ions of the solute. In those cases, the sum of the moles of molecules of solute and solvent is not actually the total moles of independent particles solution. To sidestep that problem, the solubility per mole of solution is usually computed and quoted as if the solute does not dissociate or form complexes -- that is, by pretending that the mole corporeality of solution is the sum of the mole amounts of the two substances.
Qualifiers used to depict extent of solubility
The extent of solubility ranges widely, from infinitely soluble (without limit, i. e. miscible[two]) such every bit ethanol in h2o, to essentially insoluble, such as titanium dioxide in water. A number of other descriptive terms are also used to qualify the extent of solubility for a given application. For example, U.S. Pharmacopoeia gives the following terms, according to the mass thousand sv of solvent required to deliquesce one unit of mass m su of solute:[8] (The solubilities of the examples are guess, for water at xx-25 °C.)
| Term | range | Example | g/dL | m sv/m su |
|---|---|---|---|---|
| Very soluble | <1 | calcium nitrate | 158.7 | 0.63 |
| Freely soluble | 1 to ten | calcium chloride | 65 | 1.54 |
| Soluble | 10 to xxx | sodium oxalate | 3.9 | 26 |
| Sparingly soluble | xxx to 100 | |||
| Slightly soluble | 100 to thou | calcium sulfate | 0.21 | 490 |
| Very slightly soluble | thou to 10,000 | dicalcium phosphate | 0.02 | 5000 |
| Practically insoluble or insoluble | ≥ 10,000 | barium sulfate | 0.000245 | 409000 |
The thresholds to draw something every bit insoluble, or similar terms, may depend on the application. For example, ane source states that substances are described as "insoluble" when their solubility is less than 0.1 thou per 100 mL of solvent.[9]
Molecular view
Solubility occurs nether dynamic equilibrium, which ways that solubility results from the simultaneous and opposing processes of dissolution and stage joining (e.k. precipitation of solids). The solubility equilibrium occurs when the two processes keep at equal and opposite rates.
The term solubility is also used in some fields where the solute is altered by solvolysis. For example, many metals and their oxides are said to exist "soluble in hydrochloric acid", although in fact the aqueous acid irreversibly degrades the solid to give soluble products. It is also truthful that most ionic solids are dissolved by polar solvents, but such processes are reversible. In those cases where the solute is non recovered upon evaporation of the solvent, the process is referred to every bit solvolysis. The thermodynamic concept of solubility does not utilize straightforwardly to solvolysis.
When a solute dissolves, it may class several species in the solution. For example, an aqueous suspension of ferrous hydroxide, Fe(OH)
2 , volition incorporate the series [Atomic number 26(HtwoO) 10 (OH) 10 ](2x)+ likewise as other species. Furthermore, the solubility of ferrous hydroxide and the composition of its soluble components depend on pH. In general, solubility in the solvent phase tin exist given only for a specific solute that is thermodynamically stable, and the value of the solubility will include all the species in the solution (in the example to a higher place, all the atomic number 26-containing complexes).
Factors affecting solubility
Solubility is defined for specific phases. For instance, the solubility of aragonite and calcite in h2o are expected to differ, even though they are both polymorphs of calcium carbonate and have the same chemical formula.
The solubility of i substance in another is adamant by the balance of intermolecular forces betwixt the solvent and solute, and the entropy change that accompanies the solvation. Factors such as temperature and pressure level volition alter this rest, thus changing the solubility.
Solubility may also strongly depend on the presence of other species dissolved in the solvent, for example, complex-forming anions (ligands) in liquids. Solubility will also depend on the excess or deficiency of a mutual ion in the solution, a phenomenon known as the common-ion effect. To a lesser extent, solubility will depend on the ionic strength of solutions. The terminal two effects tin be quantified using the equation for solubility equilibrium.
For a solid that dissolves in a redox reaction, solubility is expected to depend on the potential (within the range of potentials under which the solid remains the thermodynamically stable phase). For example, solubility of aureate in high-temperature water is observed to exist almost an order of magnitude higher (i.e. near x times higher) when the redox potential is controlled using a highly oxidizing Fe3O4-FetwoO3 redox buffer than with a moderately oxidizing Ni-NiO buffer.[10]
Solubility (metastable, at concentrations approaching saturation) also depends on the concrete size of the crystal or droplet of solute (or, strictly speaking, on the specific surface expanse or molar surface expanse of the solute).[11] For quantification, run across the equation in the commodity on solubility equilibrium. For highly lacking crystals, solubility may increase with the increasing caste of disorder. Both of these effects occur because of the dependence of solubility constant on the Gibbs energy of the crystal. The concluding two effects, although often difficult to mensurate, are of practical importance.[ commendation needed ] For example, they provide the driving forcefulness for precipitate aging (the crystal size spontaneously increasing with time).
Temperature
The solubility of a given solute in a given solvent is function of temperature. Depending on the modify in enthalpy (ΔH) of the dissolution reaction, i.e., on the endothermic (ΔH > 0) or exothermic (ΔH < 0) character of the dissolution reaction, the solubility of a given compound may increase or decrease with temperature. The van 't Hoff equation relates the modify of solubility equilibrium constant (One thousand sp) to temperature change and to reaction enthalpy change. For most solids and liquids, their solubility increases with temperature because their dissolution reaction is endothermic (ΔH > 0).[12] In liquid h2o at high temperatures, (e.g. that approaching the critical temperature), the solubility of ionic solutes tends to subtract due to the change of backdrop and structure of liquid water; the lower dielectric constant results in a less polar solvent and in a change of hydration energy affecting the ΔG of the dissolution reaction.
Gaseous solutes exhibit more complex behavior with temperature. As the temperature is raised, gases usually become less soluble in water (exothermic dissolution reaction related to their hydration) (to a minimum, which is beneath 120 °C for almost permanent gases[13]), but more soluble in organic solvents (endothermic dissolution reaction related to their solvation).[12]
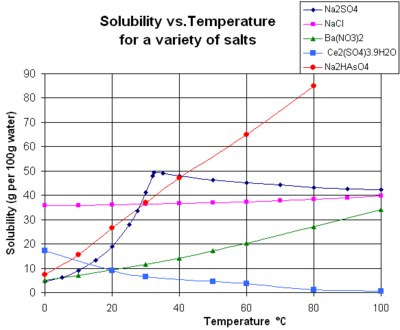
The nautical chart shows solubility curves for some typical solid inorganic salts in liquid water (temperature is in degrees Celsius, i.east. kelvins minus 273.15).[14] Many salts bear like barium nitrate and disodium hydrogen arsenate, and show a large increase in solubility with temperature (ΔH > 0). Some solutes (e.g. sodium chloride in water) showroom solubility that is fairly independent of temperature (ΔH ≈ 0). A few, such as calcium sulfate (gypsum) and cerium(3) sulfate, become less soluble in water equally temperature increases (ΔH < 0).[xv] This is too the example for calcium hydroxide (portlandite), whose solubility at 70 °C is about half of its value at 25 °C. The dissolution of calcium hydroxide in water is besides an exothermic process (ΔH < 0) and obeys the van 't Hoff equation and Le Chatelier'south principle. A lowering of temperature favors the removal of dissolution heat from the system and thus favors dissolution of Ca(OH)2: and then portlandite solubility increases at low temperature. This temperature dependence is sometimes referred to as "retrograde" or "inverse" solubility. Occasionally, a more complex design is observed, every bit with sodium sulfate, where the less soluble decahydrate crystal (mirabilite) loses h2o of crystallization at 32 °C to form a more than soluble anhydrous stage (thenardite) with a smaller modify in Gibbs free energy (ΔG) in the dissolution reaction.[ citation needed ]
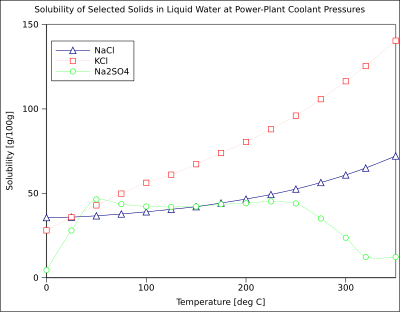
The solubility of organic compounds well-nigh always increases with temperature. The technique of recrystallization, used for purification of solids, depends on a solute's dissimilar solubilities in hot and common cold solvent. A few exceptions exist, such as certain cyclodextrins.[16]
Pressure
For condensed phases (solids and liquids), the pressure level dependence of solubility is typically weak and usually neglected in practice. Assuming an ideal solution, the dependence can be quantified as:
where the alphabetize iterates the components, is the mole fraction of the -thursday component in the solution, is the pressure, the index refers to constant temperature, is the fractional tooth book of the -th component in the solution, is the fractional tooth volume of the -th component in the dissolving solid, and is the universal gas constant.[17]
The pressure dependence of solubility does occasionally have practical significance. For case, atmospheric precipitation fouling of oil fields and wells past calcium sulfate (which decreases its solubility with decreasing pressure) can result in decreased productivity with fourth dimension.
Solubility of gases
Henry'due south law is used to quantify the solubility of gases in solvents. The solubility of a gas in a solvent is directly proportional to the fractional pressure of that gas above the solvent. This relationship is similar to Raoult'south law and tin can exist written equally:
where is a temperature-dependent abiding (for example, 769.two L·atm/mol for dioxygen (O2) in h2o at 298 K), is the partial pressure (in atm), and is the concentration of the dissolved gas in the liquid (in mol/Fifty).
The solubility of gases is sometimes besides quantified using Bunsen solubility coefficient.
In the presence of small bubbles, the solubility of the gas does not depend on the chimera radius in whatsoever other way than through the effect of the radius on force per unit area (i.e. the solubility of gas in the liquid in contact with small bubbles is increased due to force per unit area increase by Δp = 2γ/r; see Young–Laplace equation).[18]
Henry's police force is valid for gases that practice non undergo change of chemical speciation on dissolution. Sieverts' law shows a case when this assumption does not hold.
The carbon dioxide solubility in seawater is too afflicted by temperature, pH of the solution, and past the carbonate buffer. The decrease of solubility of carbon dioxide in seawater when temperature increases is also an of import retroaction factor (positive feedback) exacerbating by and future climate changes as observed in water ice cores from the Vostok site in Antarctica. At the geological fourth dimension calibration, because of the Milankovich cycles, when the astronomical parameters of the Earth orbit and its rotation axis progressively alter and modify the solar irradiance at the World surface, temperature starts to increase. When a deglaciation catamenia is initiated, the progressive warming of the oceans releases CO2 into the atmosphere considering of its lower solubility in warmer sea water. In plow, higher levels of CO2 in the temper increment the greenhouse effect and carbon dioxide acts as an amplifier of the general warming.
Polarity
A popular aphorism used for predicting solubility is "similar dissolves like" also expressed in the Latin language as "Similia similibus solventur".[19] This argument indicates that a solute will dissolve best in a solvent that has a similar chemical structure to itself, based on favorable entropy of mixing. This view is simplistic, but it is a useful rule of thumb. The overall solvation capacity of a solvent depends primarily on its polarity.[a] For example, a very polar (hydrophilic) solute such as urea is very soluble in highly polar water, less soluble in fairly polar methanol, and practically insoluble in non-polar solvents such as benzene. In contrast, a non-polar or lipophilic solute such as naphthalene is insoluble in water, fairly soluble in methanol, and highly soluble in not-polar benzene.[twenty]

Dissolution of sodium chloride in water
In even more than simple terms a simple ionic chemical compound (with positive and negative ions) such every bit sodium chloride (mutual salt) is hands soluble in a highly polar solvent (with some separation of positive (δ+) and negative (δ-) charges in the covalent molecule) such every bit h2o, every bit thus the sea is salty as it accumulates dissolved salts since early geological ages.
The solubility is favored by entropy of mixing (ΔSouth) and depends on enthalpy of dissolution (ΔH) and the hydrophobic result. The complimentary energy of dissolution (Gibbs free energy) depends on temperature and is given by the human relationship: ΔYard = ΔH – TΔS. Smaller ΔM ways greater solubility.
Chemists often exploit differences in solubilities to divide and purify compounds from reaction mixtures, using the technique of liquid-liquid extraction. This applies in vast areas of chemical science from drug synthesis to spent nuclear fuel reprocessing.
Charge per unit of dissolution
Dissolution is non an instantaneous process. The rate of solubilization (in kg/southward) is related to the solubility product and the area of the fabric. The speed at which a solid dissolves may depend on its crystallinity or lack thereof in the instance of amorphous solids and the surface area (crystallite size) and the presence of polymorphism. Many practical systems illustrate this effect, for example in designing methods for controlled drug commitment. In some cases, solubility equilibria can take a long fourth dimension to establish (hours, days, months, or many years; depending on the nature of the solute and other factors).
The charge per unit of dissolution tin can be often expressed by the Noyes–Whitney equation or the Nernst and Brunner equation[21] of the class:
where:
For dissolution limited by improvidence (or mass transfer if mixing is present), is equal to the solubility of the substance. When the dissolution charge per unit of a pure substance is normalized to the surface area of the solid (which ordinarily changes with fourth dimension during the dissolution procedure), then information technology is expressed in kg/mtwos and referred to as "intrinsic dissolution rate". The intrinsic dissolution rate is defined past the United States Pharmacopeia.
Dissolution rates vary past orders of magnitude between different systems. Typically, very low dissolution rates parallel low solubilities, and substances with high solubilities exhibit high dissolution rates, as suggested by the Noyes-Whitney equation.
Theories of solubility
Solubility product
Solubility constants are used to draw saturated solutions of ionic compounds of relatively depression solubility (see solubility equilibrium). The solubility constant is a special case of an equilibrium abiding. Since it is a product of ion concentrations in equilibrium, it is also known equally the solubility product. It describes the balance betwixt dissolved ions from the table salt and undissolved table salt. The solubility constant is also "applicable" (i.eastward. useful) to precipitation, the contrary of the dissolving reaction. As with other equilibrium constants, temperature tin can affect the numerical value of solubility constant. While the solubility constant is not as elementary every bit solubility, the value of this constant is generally independent of the presence of other species in the solvent.
Other theories
The Flory–Huggins solution theory is a theoretical model describing the solubility of polymers. The Hansen solubility parameters and the Hildebrand solubility parameters are empirical methods for the prediction of solubility. Information technology is also possible to predict solubility from other physical constants such as the enthalpy of fusion.
The octanol-water sectionalization coefficient, usually expressed as its logarithm (Log P), is a measure of differential solubility of a compound in a hydrophobic solvent (1-octanol) and a hydrophilic solvent (h2o). The logarithm of these 2 values enables compounds to exist ranked in terms of hydrophilicity (or hydrophobicity).
The energy change associated with dissolving is unremarkably given per mole of solute as the enthalpy of solution.
Applications
Solubility is of fundamental importance in a large number of scientific disciplines and practical applications, ranging from ore processing and nuclear reprocessing to the use of medicines, and the send of pollutants.
Solubility is often said to be one of the "characteristic properties of a substance", which ways that solubility is commonly used to depict the substance, to indicate a substance'due south polarity, to assistance to distinguish it from other substances, and every bit a guide to applications of the substance. For example, indigo is described as "insoluble in water, alcohol, or ether but soluble in chloroform, nitrobenzene, or concentrated sulfuric acid".[ citation needed ]
Solubility of a substance is useful when separating mixtures. For example, a mixture of table salt (sodium chloride) and silica may be separated by dissolving the table salt in water, and filtering off the undissolved silica. The synthesis of chemical compounds, by the milligram in a laboratory, or by the ton in manufacture, both brand use of the relative solubilities of the desired product, besides equally unreacted starting materials, byproducts, and side products to achieve separation.
Another example of this is the synthesis of benzoic acid from phenylmagnesium bromide and dry ice. Benzoic acid is more soluble in an organic solvent such as dichloromethane or diethyl ether, and when shaken with this organic solvent in a separatory funnel, will preferentially deliquesce in the organic layer. The other reaction products, including the magnesium bromide, will remain in the aqueous layer, clearly showing that separation based on solubility is achieved. This process, known as liquid–liquid extraction, is an important technique in constructed chemistry. Recycling is used to ensure maximum extraction.
Differential solubility
In flowing systems, differences in solubility ofttimes determine the dissolution-precipitation driven transport of species. This happens when dissimilar parts of the organization feel different conditions. Even slightly different conditions tin can result in significant furnishings, given sufficient fourth dimension.
For case, relatively low solubility compounds are plant to be soluble in more extreme environments, resulting in geochemical and geological furnishings of the activity of hydrothermal fluids in the Earth'due south chaff. These are often the source of high quality economical mineral deposits and precious or semi-precious gems. In the same manner, compounds with low solubility will deliquesce over extended time (geological time), resulting in significant effects such every bit extensive cave systems or Karstic land surfaces.
Solubility of ionic compounds in h2o
Some ionic compounds (salts) dissolve in h2o, which arises considering of the attraction between positive and negative charges (meet: solvation). For example, the common salt'south positive ions (e.thou. Ag+) attract the partially negative oxygen atom in H2O. Likewise, the salt'due south negative ions (e.m. Cl−) attract the partially positive hydrogens in H2O. Note: the oxygen cantlet is partially negative because it is more electronegative than hydrogen, and vice versa (see: chemical polarity).
- AgCl(southward) ⇌ Ag+ (aq) + Cl− (aq)
Yet, there is a limit to how much salt can be dissolved in a given book of water. This concentration is the solubility and related to the solubility product, K sp. This equilibrium constant depends on the type of table salt (AgCl vs. NaCl, for case), temperature, and the common ion issue.
One can calculate the amount of AgCl that will dissolve in 1 liter of pure water as follows:
- K sp = [Ag+] × [Cl−] / M2 (definition of solubility product; M = mol/L)
- K sp = 1.eight × x−10 (from a table of solubility products)
[Ag+] = [Cl−], in the absence of other silverish or chloride salts, and so
- [Ag+]2 = 1.eight × 10−10 M2
- [Ag+] = 1.34 × ten−v mol/L
The upshot: 1 liter of water can dissolve ane.34 × x−5 moles of AgCl at room temperature. Compared with other salts, AgCl is poorly soluble in water. For instance, salt (NaCl) has a much higher K sp = 36 and is, therefore, more soluble. The following table gives an overview of solubility rules for various ionic compounds.
| Soluble | Insoluble[22] |
|---|---|
| Group I and NH4 + compounds (except lithium phosphate) | Carbonates (except Grouping I, NHfour + and uranyl compounds) |
| Nitrates | Sulfites (except Group I and NHfour + compounds) |
| Acetates (ethanoates) (except Ag+ compounds) | Phosphates (except Grouping I and NH4 + compounds (excluding Li+)) |
| Chlorides (chlorates and perchlorates), bromides and iodides (except Ag+, Pbtwo+, Cu+ and Hgtwo 2+) | Hydroxides and oxides (except Group I, NH4 +, Ba2+, Srtwo+ and Tl+) |
| Sulfates (except Ag+, Pbtwo+, Ba2+, Srii+ and Ca2+) | Sulfides (except Group I, Grouping II and NH4 + compounds) |
Solubility of organic compounds
The principle outlined above under polarity, that like dissolves like, is the usual guide to solubility with organic systems. For example, petroleum jelly will dissolve in gasoline because both petroleum jelly and gasoline are non-polar hydrocarbons. Information technology volition not, on the other hand, dissolve in ethyl alcohol or water, since the polarity of these solvents is too high. Sugar will not dissolve in gasoline, since saccharide is too polar in comparison with gasoline. A mixture of gasoline and carbohydrate tin can therefore be separated by filtration or extraction with h2o.
Solid solution
This term is frequently used in the field of metallurgy to refer to the extent that an alloying element will dissolve into the base metal without forming a separate phase. The solvus or solubility line (or curve) is the line (or lines) on a phase diagram that give the limits of solute add-on. That is, the lines show the maximum amount of a component that can exist added to some other component and nonetheless be in solid solution. In the solid's crystalline structure, the 'solute' element can either take the identify of the matrix within the lattice (a substitutional position; for example, chromium in iron) or take a place in a space between the lattice points (an interstitial position; for case, carbon in iron).
In microelectronic fabrication, solid solubility refers to the maximum concentration of impurities ane can identify into the substrate.
In solid compounds (as opposed to elements), the solubility of a solute element can too depend on the phases separating out in equilibrium. For instance, amount of Sn soluble in the ZnSb phase can depend significantly on whether the phases separating out in equilibrium are (ZnfourSb3+Sn(L)) or (ZnSnSb2+Sn(L))[23]. Besides these, the ZnSb compound with Sn as a solute can separate out into other combinations of phases after the solubility limit is reached depending on the initial chemical composition during synthesis. Each combination produces a different solubility of Sn in ZnSb. Hence solubility studies in compounds, concluded upon the get-go instance of observing secondary phases separating out might underestimate solubility.[24] While the maximum number of phases separating out at once in equilibrium can be determined by the Gibb'south phase rule, for chemic compounds at that place is no limit on the number of such phase separating combinations itself. Hence, establishing the "maximum solubility" in solid compounds experimentally can be difficult, requiring equilibration of many samples. If the dominant crystallographic defect (by and large interstitial or substitutional point defects) involved in the solid-solution can be chemically intuited beforehand, then using some simple thermodynamic guidelines can considerably reduce the number of samples required to establish maximum solubility. [25]
Incongruent dissolution
Many substances dissolve congruently (i.e. the composition of the solid and the dissolved solute stoichiometrically match). However, some substances may dissolve incongruently, whereby the composition of the solute in solution does non match that of the solid. This solubilization is accompanied by amending of the "master solid" and possibly germination of a secondary solid phase. However, in general, some primary solid besides remains and a complex solubility equilibrium establishes. For example, dissolution of albite may result in formation of gibbsite.[26]
- NaAlSiiiiO8(s) + H+ + 7H2O ⇌ Na+ + Al(OH)3(s) + 3HivSiO4 .
In this case, the solubility of albite is expected to depend on the solid-to-solvent ratio. This kind of solubility is of cracking importance in geology, where information technology results in germination of metamorphic rocks.
In principle, both congruent and incongruent dissolution can atomic number 82 to the formation of secondary solid phases in equilibrium. So, in the field of Materials Science, the solubility for both cases is described more generally on chemic limerick phase diagrams.
Solubility prediction
Solubility is a property of interest in many aspects of scientific discipline, including merely not limited to: environmental predictions, biochemistry, chemist's, drug-pattern, agrochemical design, and protein ligand binding. Aqueous solubility is of fundamental involvement owing to the vital biological and transportation functions played by h2o.[27] [28] [29] In addition, to this articulate scientific involvement in water solubility and solvent effects; accurate predictions of solubility are of import industrially. The power to accurately predict a molecule's solubility represents potentially large financial savings in many chemical product development processes, such every bit pharmaceuticals.[30] In the pharmaceutical industry, solubility predictions form part of the early stage lead optimisation process of drug candidates. Solubility remains a business organisation all the way to formulation.[30] A number of methods have been applied to such predictions including quantitative structure–activity relationships (QSAR), quantitative structure–property relationships (QSPR) and information mining. These models provide efficient predictions of solubility and represent the current standard. The draw back such models is that they can lack physical insight. A method founded in concrete theory, capable of achieving similar levels of accurateness at an sensible cost, would be a powerful tool scientifically and industrially.[31] [32] [33] [34]
Methods founded in physical theory tend to use thermodynamic cycles, a concept from classical thermodynamics. The two common thermodynamic cycles used involve either the calculation of the complimentary energy of sublimation (solid to gas without going through a liquid state) and the free free energy of solvating a gaseous molecule (gas to solution), or the complimentary free energy of fusion (solid to a molten phase) and the free free energy of mixing (molten to solution). These two process are represented in the following diagrams.

Thermodynamic bicycle for calculating solvation via sublimation

Thermodynamic cycle for computing solvation via fusion
These cycles accept been used for attempts at first principles predictions (solving using the fundamental physical equations) using physically motivated solvent models,[32] to create parametric equations and QSPR models[35] [33] and combinations of the ii.[33] The use of these cycles enables the calculation of the solvation free energy indirectly via either gas (in the sublimation wheel) or a cook (fusion cycle). This is helpful as calculating the costless energy of solvation directly is extremely difficult. The costless energy of solvation tin can be converted to a solubility value using diverse formulae, the most general case being shown below, where the numerator is the free free energy of solvation, R is the gas constant and T is the temperature in kelvins.[32]
Well known fitted equations for solubility prediction are the general solubility equations. These equations stem from the work of Yalkowsky et al.[36] [37] The original formula is given first, followed by a revised formula which takes a dissimilar assumption of complete miscibility in octanol.[37]
These equations are founded on the principles of the fusion cycle.
See also
- Apparent molar property
- Biopharmaceutics Classification Arrangement
- Dühring's rule
- Fajans–Paneth–Hahn Constabulary
- Flexible SPC water model
- Henry'due south law – Relation of equilibrium solubility of a gas in a liquid to its partial pressure in the contacting gas phase
- Hot water extraction
- Hydrotrope
- Micellar solubilization – Procedure of incorporating the solubilizate into or onto micelles
- Raoult's law – Police force of thermodynamics for vapour pressure of a mixture
- Charge per unit of solution
- Solubility equilibrium
- van 't Hoff equation – Relation between temperature and the equilibrium constant of a chemic reaction
Notes
- ^ The solvent polarity is divers as its solvation power according to Reichardt.
References
- ^ a b IUPAC, Compendium of Chemic Terminology, 2nd ed. (the "Golden Book") (1997). Online corrected version: (2006–) "Solubility". doi:10.1351/goldbook.S05740
- ^ a b Clugston, M.; Fleming, R. (2000). Advanced Chemical science (1st ed.). Oxford: Oxford Publishing. p. 108.
- ^ J. de Swaan Arons and Yard. A. Yard. Diepen (1966): "Gas—Gas Equilibria". Journal of Chemic Physics, volume 44, upshot 6, page 2322. doi:10.1063/one.1727043
- ^ Tomlinson, Charles (1868-01-01). "On Supersaturated Saline Solutions". Philosophical Transactions of the Regal Lodge of London. 158: 659–673. doi:10.1098/rstl.1868.0028. ISSN 0261-0523. S2CID 110079029.
- ^ Claudius Kormann, Detlef W. Bahnemann, and Michael R. Hoffmann (1988): "Training and label of breakthrough-size titanium dioxide". Periodical of Physical Chemistry,volume 92, issue xviii, pages 5196–5201. doi:10.1021/j100329a027
- ^ Abler (2021): "Westward/W (Weight/Weight)". Online folio at Abler.com website. Accessed on 2021-11-26.
- ^ I. Lee and J. Lee (2012): "Measurement of mixing ratio and volume change of ethanol-h2o binary mixtures using suspended microchannel resonators." SENSORS, book 2012, pages one-iii. doi:ten.1109/ICSENS.2012.6411272.
- ^ "Pharmacopeia of the The states, 32nd revision, and the National Formulary, 27th edition," 2009, pp.1 to 12.
- ^
- ^ I.Y. Nekrasov (1996). Geochemistry, Mineralogy and Genesis of Gold Deposits. Taylor & Francis. pp. 135–136. ISBN978-90-5410-723-1.
- ^ Hefter, G.T.; Tomkins, R.P.T (Editors) (2003). The Experimental Determination of Solubilities. Wiley-Blackwell. ISBN978-0-471-49708-0.
- ^ a b John W. Hill, Ralph H. Petrucci, General Chemistry, 2nd edition, Prentice Hall, 1999.
- ^ P. Cohen, ed. (1989). The ASME Handbook on Water Technology for Thermal Power Systems. The American Society of Mechanical Engineers. p. 442.
- ^ Handbook of Chemistry and Physics (27th ed.). Cleveland, Ohio: Chemical Rubber Publishing Co. 1943.
- ^ "What substances, such equally cerium sulfate, accept a lower solubility when they are heated?". Retrieved 28 May 2014.
- ^ Salvatore Filippone, Frank Heimanna and André Rassat (2002). "A highly water-soluble 2+1 b-cyclodextrin–fullerene conjugate". Chem. Commun. 2002 (14): 1508–1509. doi:10.1039/b202410a.
- ^ E.1000. Gutman (1994). Mechanochemistry of Solid Surfaces. World Scientific Publishing Co.
- ^ Thou.West. Greenwood (1969). "The Solubility of Gas Bubbles". Journal of Materials Science. four (4): 320–322. Bibcode:1969JMatS...4..320G. doi:10.1007/BF00550401.
- ^ Kenneth J. Williamson (1994). Macroscale and Microscale Organic Experiments (2nd ed.). Lexington, Massachusetts: D. C, Heath. p. 40. ISBN978-0-669-19429-6.
- ^ Merck Index (7th ed.). Merck & Co. 1960.
- ^ Dokoumetzidis, Aristides; Macheras, Panos (2006). "A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System". Int. J. Pharm. 321 (1–2): 1–xi. doi:10.1016/j.ijpharm.2006.07.011. PMID 16920290.
- ^ C. Houk; R. Post, eds. (1997). Chemical science, Concept and Problems . John Wiley & Sons. p. 121. ISBN978-0-471-12120-half-dozen.
- ^ Wood, Maxwell; Toriyama, Michael; Dugar, Shristi; Male person, James; Anand, Shashwat; Stevanović, Vladan; Snyder, Jeff (2020). "Phase Boundary Mapping of Can-Doped ZnSb Reveals Thermodynamic Route to Loftier Thermoelectric Efficiency". Avant-garde Energy Materials. 11 (twenty). doi:ten.1002/aenm.202100181.
- ^ Tang, Yinglu; Hanus, Riley; Chen, Sin-wen; Snyder, Jeff (2015). "Solubility blueprint leading to high figure of merit in depression-cost Ce-CoSb3 skutterudites". Nature Communications. 6 (7584). doi:10.1038/ncomms8584.
- ^ Anand, Shashwat; Wolverton, Chris; Snyder, Jeff (2022). "Thermodynamic Guidelines for Maximum Solubility". Chemistry of Materials. 34 (4): 1638–1648. doi:10.1021/acs.chemmater.1c03715.
- ^ O.M. Saether; P. de Caritat, eds. (1997). Geochemical processes, weathering and groundwater recharge in catchments. Rotterdam: Taylor & Francis. p. 6. ISBN978-90-5410-641-eight.
- ^ Skyner, R.; McDonagh, J. L.; Groom, C. R.; van Mourik, T.; Mitchell, J. B. O. (2015). "A Review of Methods for the Adding of Solution Complimentary Energies and the Modelling of Systems in Solution" (PDF). Phys Chem Chem Phys. 17 (9): 6174–91. Bibcode:2015PCCP...17.6174S. doi:10.1039/C5CP00288E. PMID 25660403.
- ^ Tomasi, J.; Mennucci, B.; Cammi, R. (2005). "Quantum Mechanical Continuum Solvation Models". Chemical Reviews. 105 (viii): 2999–3093. doi:10.1021/cr9904009. PMID 16092826.
- ^ Cramer, C. J.; Truhlar, D. G. (1999). "Implicit Solvation Models: Equilibria, Structure, Spectra, and Dynamics". Chemical Reviews. 99 (8): 2161–2200. doi:10.1021/cr960149m. PMID 11849023.
- ^ a b Abramov, Y. A. (2015). "Major Source of Fault in QSPR Prediction of Intrinsic Thermodynamic Solubility of Drugs: Solid vs Nonsolid Land Contributions?". Molecular Pharmaceutics. 12 (half dozen): 2126–2141. doi:ten.1021/acs.molpharmaceut.5b00119. PMID 25880026.
- ^ McDonagh, J. 50. (2015). Computing the Aqueous solubility of Organic Drug-Like Molecules and Understanding Hydrophobicity. University of St Andrews. hdl:10023/6534.
- ^ a b c Palmer, D. Due south.; McDonagh, J. 50.; Mitchell, J. B. O.; van Mourik, T.; Fedorov, Chiliad. V. (2012). "First-Principles Calculation of the Intrinsic Aqueous Solubility of Crystalline Druglike Molecules". Journal of Chemical Theory and Ciphering. 8 (9): 3322–3337. doi:10.1021/ct300345m. PMID 26605739.
- ^ a b c McDonagh, J. Fifty.; Nath, Due north.; De Ferrari, L.; van Mourik, T.; Mitchell, J. B. O. (2014). "Uniting Cheminformatics and Chemical Theory To Predict the Intrinsic Aqueous Solubility of Crystalline Druglike Molecules". Journal of Chemic Information and Modeling. 54 (iii): 844–856. doi:10.1021/ci4005805. PMC3965570. PMID 24564264.
- ^ Lusci, A.; Pollastri, G.; Baldi, P. (2013). "Deep Architectures and Deep Learning in Chemoinformatics: The Prediction of Aqueous Solubility for Drug-Like Molecules". Periodical of Chemic Information and Modeling. 53 (7): 1563–1575. doi:x.1021/ci400187y. PMC3739985. PMID 23795551.
- ^ Ran, Y.; N. Jain; Due south.H. Yalkowsky (2001). "Prediction of Aqueous Solubility of Organic Compounds by the Full general Solubility Equation (GSE)". Periodical of Chemical Information and Modeling. 41 (5): 1208–1217. doi:10.1021/ci010287z.
- ^ Yalkowsky, Southward.H.; Valvani, S.C. (1980). "Solubility and partitioning I: solubility of nonelectrolytes in h2o". Journal of Pharmaceutical Sciences. 69 (8): 912–922. doi:ten.1002/jps.2600690814. PMID 7400936.
- ^ a b Jain, N.; Yalkowsky, S.H. (2001). "Estimation of the aqueous solubility I: application to organic nonelectrolytes". Journal of Pharmaceutical Sciences. 90 (2): 234–252. doi:ten.1002/1520-6017(200102)90:2<234::aid-jps14>three.0.co;2-v.
External links
Source: https://en.wikipedia.org/wiki/Solubility

















0 Response to "what is the ability of a substance to dissolve in another substance?"
Post a Comment